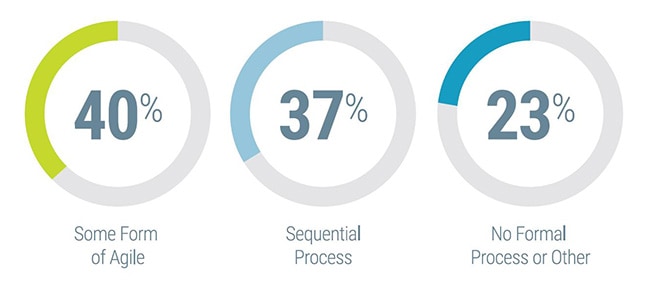
Recent industry discussions have pointed to increased utilization of agile methodologies for in vitro diagnostics (IVD) instrument development, with a number of IVD product developers indicating that they were in the early stages of transitioning from traditional product development methods to more of an agile approach. However, there had not been a formalized study exploring the degree to which this was the case.
Having adopted agile methodologies for IVD instrument development projects and seeing positive results, Invetech was interested in assessing the degree to which the IVD industry as a whole has begun to embrace agile beyond the software team.
Working with Hanover Research, a global research and analytics firm, a blinded survey was administered to diagnostics and life science instrument developers to investigate the adoption and usage of agile methodologies at these companies, as well as some of the associated challenges and benefits. The survey was distributed by the American Association for Clinical Chemistry (AACC), generating a total of 90 qualified industry responses.
Below is an executive summary of the key findings from the survey to serve as a reference for IVD manufacturers to benchmark agile adoption.
Download the full benchmarking report for a compilation of all the survey findings.
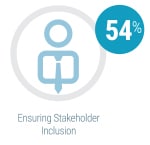 By far the most significant contributing factor in successful agile adoption was ensuring stakeholder inclusion from the very start of the process, cited by 54 percent of respondents currently using agile methods. The second most frequently cited key success factor was co-locating team members (31 percent). Identifying a leader, using visual management, and starting with smaller projects were also noted as contributing to agile success, each cited by 19 percent.
By far the most significant contributing factor in successful agile adoption was ensuring stakeholder inclusion from the very start of the process, cited by 54 percent of respondents currently using agile methods. The second most frequently cited key success factor was co-locating team members (31 percent). Identifying a leader, using visual management, and starting with smaller projects were also noted as contributing to agile success, each cited by 19 percent.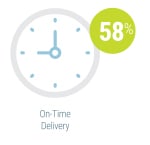 On-time delivery (58 percent) and product quality (54 percent) were the two most frequently cited metrics used by respondents to measure the success of instrument development programs using agile methodologies. Team productivity and business value were also considered important success metrics, each cited by 35 percent of survey participants.
On-time delivery (58 percent) and product quality (54 percent) were the two most frequently cited metrics used by respondents to measure the success of instrument development programs using agile methodologies. Team productivity and business value were also considered important success metrics, each cited by 35 percent of survey participants.